
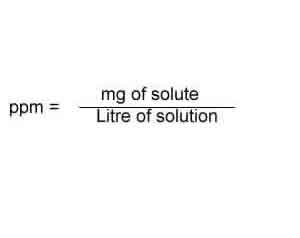
Everyone has a particular view of what is meant by the concentration of a solution. They may also talk about the concentration of coffee or tea. As an example, to represent concentration of aqueous HCl, we can useĮxample: = 0.1 mol dm-3 What Is Concentration Of SolutionĮveryone talks about the concentration of solutions. Sometimes we use ” to represent concentration of solute. If 10 dm3 aqueous solution contains 0.98 mol of HNO3, molarity of HNO3 is 0.098 mol dm-3.Otherwise, we can describe this is as, 0.45 mol of HCl is dissolved 1 dm3 of the total solution. If 1 dm3 aqueous solution contains 0.45 mol of HCl, molarity of HCl is 0.45 mol dm-3.The determination of the concentration of hydroxide ions and pOH will be later used to show the relationship between pH and pOH.Īlso Check: Michael Jackson Kids Biological Parents Examples About Molarity Values How to Calculate pH explains its categories the scientific mathematics and role pH has in our lives. Thank you for your patience!Ībstract: pH is a unit of measurement often used in fundamental chemistry concepts. We are gradually updating these posts and will remove this disclaimer when this post is updated. How To Calculate Ph In ChemistryĪttention: This post was written a few years ago and may not reflect the latest changes in the AP® program. Therefore, a plot of versus t will always yield a straight line with a slope of -\text. Note that this equation has the form \text=\text. This is the integrated rate law for a zero-order reaction. Recall that the rate of a chemical reaction is defined in terms of the change in concentration of a reactant per change in time. Plot Of Concentration Versus Time For A Zero The image shows a solution from the most dilute solution to the most concentrated solution. Now that you understand the concept of what is concentration of solution let’s move on to the different methods of expressing concentration. Whereas when the solution has more solvent in it, we call it a dilute solution. When a solution has more solute in it, we call it a concentrated solution.
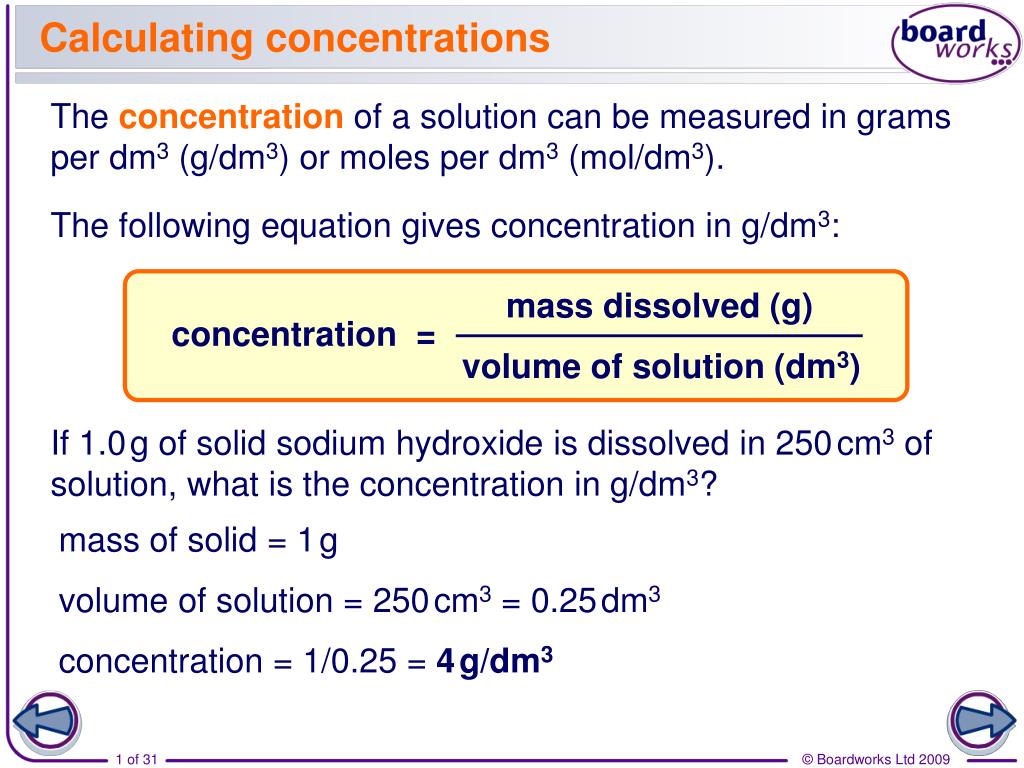
In chemistry, we define concentration of solution as the amount of solute in a solvent. The amount of solute in the solvent is what is called the concentration of a solution. We always need to keep an account of the amount of solute in the solution. They are the two basic solution concentration terms that you need to know. In an aqueous solution, two parts exist, namely solute and solvent. Concentration Formula & Calculations | Chemical Calculations | Chemistry | Fuse School


 0 kommentar(er)
0 kommentar(er)
